Research
Synthetic Carbon Allotropes
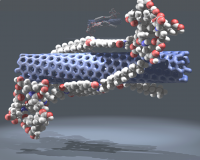
Synthetic carbon allotropes (SCAs) such as fullerenes, carbon nanotubes and graphene are characterized by a number of outstanding and unprecedented properties such as electrical conductivity, mechanical stability and transparency. They are often regarded to be among the most promising candidates for future high performance materials. To reach this challenging goal, chemical functionalization of the parent systems will play a key role. Covalent and non-covalent transformations of SCA can considerably improve and tailor their solubility and processibility and combine their properties with those of other compound classes. For this purpose we are exploring the fundamental chemical properties of SCAs and determine, for example, reaction mechanisms, selectivities of transformations and characteristic addition patterns. Binding of a large variety of organic building blocks including dendrimers, porphyrines, rylenes, sugars and peptides has been accomplished. Using our knowledge in wet-chemical functionalization of fullerenes, carbon nanotubes and graphene; new molecular architectures with remarkable functions such as photo-induced electron transfer, superoxide dismutation and protein simulation have been created.
Selected Publications
Publikationen
 C60-Trisaddukte: Fullerenophosphate mit einem C3-symmetrischen e,e,e-Trisadditionsmuster wurden regioselektiv synthetisiert. In- (siehe Bild; rot O, gelb P, blau C) und out-Invertomere bezüglich der Orientierung der P=O-Gruppe relativ zum Fullerenkern wurden isoliert und ihre Strukturen durch Röntgenstrukturanalyse bestätigt.
C60-Trisaddukte: Fullerenophosphate mit einem C3-symmetrischen e,e,e-Trisadditionsmuster wurden regioselektiv synthetisiert. In- (siehe Bild; rot O, gelb P, blau C) und out-Invertomere bezüglich der Orientierung der P=O-Gruppe relativ zum Fullerenkern wurden isoliert und ihre Strukturen durch Röntgenstrukturanalyse bestätigt.
[nsbp]
 The reactivity of reduced single walled carbon nanotubes (SWCNTs) (carbon nanotubides), prepared under strict inert conditions in a glovebox with respect to the covalent functionalization with hexyl iodide and subsequent exposure to ambient conditions (air, moisture), was systematically investigated by Raman, absorption, fluorescence, and IR spectroscopy as well as by TG/MS measurements. We have discovered that the alkylation does not lead to a complete discharging of the tubes since follow-up reactions with moisture still take place leading to mixed functionalized carbon nanotube derivatives containing H- and OH-addends (but no carboxylates) next to the hexyl groups.
The reactivity of reduced single walled carbon nanotubes (SWCNTs) (carbon nanotubides), prepared under strict inert conditions in a glovebox with respect to the covalent functionalization with hexyl iodide and subsequent exposure to ambient conditions (air, moisture), was systematically investigated by Raman, absorption, fluorescence, and IR spectroscopy as well as by TG/MS measurements. We have discovered that the alkylation does not lead to a complete discharging of the tubes since follow-up reactions with moisture still take place leading to mixed functionalized carbon nanotube derivatives containing H- and OH-addends (but no carboxylates) next to the hexyl groups.
[nsbp]
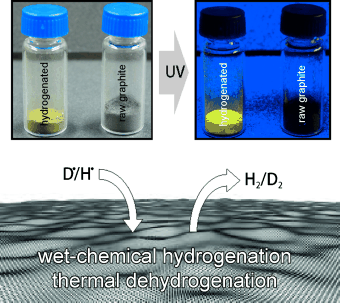 Chemistry meets graphane: a Birch-type reaction using frozen water as a gentle proton source causes the exfoliation of graphite and formation of hydrogenated graphene with electronically decoupled π-nanodomains. This highly functionalized graphene displays pronounced fluorescence.
Chemistry meets graphane: a Birch-type reaction using frozen water as a gentle proton source causes the exfoliation of graphite and formation of hydrogenated graphene with electronically decoupled π-nanodomains. This highly functionalized graphene displays pronounced fluorescence.
[nsbp]
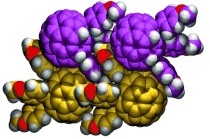 Give me five! The title compounds were isolated from the acid-catalyzed reaction of a C59N precursor with electron-rich aromatic compounds. Single-crystal X-ray diffraction on two compounds reveals characteristic packing motifs; the triaryldihydro derivative has a pseudo-stacked arrangement (C violet/yellow, N blue, O red, H white).
Give me five! The title compounds were isolated from the acid-catalyzed reaction of a C59N precursor with electron-rich aromatic compounds. Single-crystal X-ray diffraction on two compounds reveals characteristic packing motifs; the triaryldihydro derivative has a pseudo-stacked arrangement (C violet/yellow, N blue, O red, H white).
[nsbp]
Graphene, a truly two-dimensional and fully π-conjugated honeycomb carbon network, is currently evolving into the most promising successor to silicon in micro- and nanoelectronic applications. However, its wider application is impeded by the difficulties in opening a bandgap in its gapless band-structure, as well as the lack of processability in the resultant intrinscially insoluble material. Covalent chemical modification of the π-electron system is capable of addressing both of these issues through the introduction of variable chemical decoration. Although there has been significant research activity in the field of functionalized graphene, most work to date has focused on the use of graphene oxide. In this Article, we report on the first wet chemical bulk functionalization route beginning with pristine graphite that does not require initial oxidative damage of the graphene basal planes. Through effective reductive activation, covalent functionalization of the charged graphene is achieved by organic diazonium salts. Functionalization was observed spectroscopically, and successfully prevents reaggregation while providing solubility in common organic media.
[nsbp]

Twenty-five years on from the discovery of C60, the outstanding properties and potential applications of the synthetic carbon allotropes — fullerenes, nanotubes and graphene — overwhelmingly illustrate their unique scientific and technological importance.
Carbon is the element in the periodic table that provides the basis for life on Earth. It is also important for many technological applications, ranging from drugs to synthetic materials. This role is a consequence of carbon's ability to bind to itself and to nearly all elements in almost limitless variety. The resulting structural diversity of organic compounds and molecules is accompanied by a broad range of chemical and physical properties. The tools of modern synthetic chemistry allow the tailored design of these properties by the controlled combination of structural and functional building blocks in new target systems.
[nsbp]
Single-wall carbon nanotubes (SWNTs) are emerging as materials with much potential in several disciplines, in particular in electronics and photovoltaics. The combination of SWNTs with electron donors or acceptors generates active materials, which can produce electrical energy when irradiated. However, SWNTs are very elusive species when characterization of their metastable states is required. This problem mainly arises because of the polydispersive nature of SWNT samples and the inevitable presence of SWNTs in bundles of different sizes. Here, we report the complete and thorough characterization of an SWNT radical ion-pair state induced by complexation with a perylene dye, which combines excellent electron-accepting and -conducting features with a five-fused ring π-system. At the same time, the perylene dye enables the dispersion of SWNTs by means of π–π interactions, which gives individual SWNTs in solution. This work clears a path towards electronic and optoelectronic devices in which regulated electrical transport properties are important.
[nsbp]
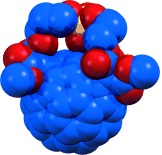
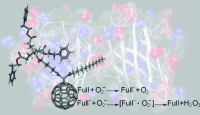 Fullerene antioxidants: A direct correlation between the redox and structural properties of water-soluble fullerenes (see picture) and their reactivity towards superoxide is demonstrated. Some of the C60 monoadducts examined act as superoxide dismutase mimetics. The findings establish a guide for designing fullerene derivatives to catalytically decompose the superoxide radical anion.
Fullerene antioxidants: A direct correlation between the redox and structural properties of water-soluble fullerenes (see picture) and their reactivity towards superoxide is demonstrated. Some of the C60 monoadducts examined act as superoxide dismutase mimetics. The findings establish a guide for designing fullerene derivatives to catalytically decompose the superoxide radical anion.
[nsbp]
Polycyclic Aromatic Hydrocarbons
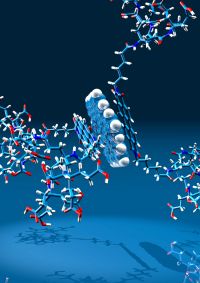
Polycyclic aromatic hydrocarbons such as hexabenzocoronenes and rylenes (naphtylenes, perylenes) do not only represent partial structures of – and model compounds for – graphene; they also represent attractive building blocks for new molecular architectures. We are particularly interested in using them as chromophores, as redox-active components and as structure determining tectons for functional hybrid molecules. Other compound classes that we are extensively investigating with respect to new derivatization concepts are porpyhrine based molecules and calixarenes. Among our accomplishments in the chemistry of all these conjugated π-systems are the first examples of water-soluble perylenes and their use for non-covalent graphene functionalization, dendritic metalloporphyines as heme-proteine models and amphiphilic calixarenes as building blocks for the first shape persistent micelles.
Selected Publications
Publikationen
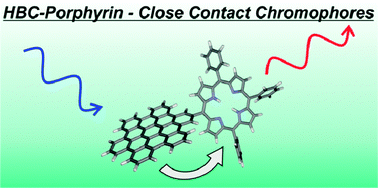
The new synthetic carbon allotrope graphene has recently attracted much attention due to its unprecedented physical properties. Since graphene is a zero band-gap semiconductor, proper functionalization and/or modification of its structure is mandatory to open a band-gap, which represents an important prerequisite for many possible applications in the field of molecular electronics. In this regard, covalent attachment of photoactive molecules is a challenging subject. Next to the introduction of a band-gap such a chemical modification would also be associated with the development of interesting photo/redox activity profiles.
[nsbp]
 On the basis of our experience with fullerenes and carbon nanotubes, we have described a series of covalent and noncovalent approaches to generate graphene derivatives. Using water-soluble perylene surfactants, we could efficiently exfoliate graphite in water and prepare substantial amounts of single-layer-graphene (SLG) and few-layer-graphene (FLG). At the same time, this approach leads to noncovalent graphene derivatives because it establishes efficient π–π-stacking interactions between graphene and the aromatic perylene chromophors supported by hydrophobic interactions.
On the basis of our experience with fullerenes and carbon nanotubes, we have described a series of covalent and noncovalent approaches to generate graphene derivatives. Using water-soluble perylene surfactants, we could efficiently exfoliate graphite in water and prepare substantial amounts of single-layer-graphene (SLG) and few-layer-graphene (FLG). At the same time, this approach leads to noncovalent graphene derivatives because it establishes efficient π–π-stacking interactions between graphene and the aromatic perylene chromophors supported by hydrophobic interactions.
[nsbp]
 As a suitable electron donor, π-extended tetrathiafulvalene (exTTF) stands out owing to its recognition of SWCNT through π–π stacking and electron donor–acceptor interactions. Herein, we explore the shape and electronic complementarity between different types of carbon nanotubes (CNT) and a tweezers-shaped molecule endowed with two exTTFs in water. The efficient electronic communication between semiconducting SWCNT/multiwall carbon nanotubes (MWCNT), on one hand, and the water-soluble exTTF nanotweezers 8, on the other hand, has been demonstrated in the ground and excited state by using steady-state as well as time-resolved spectroscopies, which were further complemented by microscopy.
As a suitable electron donor, π-extended tetrathiafulvalene (exTTF) stands out owing to its recognition of SWCNT through π–π stacking and electron donor–acceptor interactions. Herein, we explore the shape and electronic complementarity between different types of carbon nanotubes (CNT) and a tweezers-shaped molecule endowed with two exTTFs in water. The efficient electronic communication between semiconducting SWCNT/multiwall carbon nanotubes (MWCNT), on one hand, and the water-soluble exTTF nanotweezers 8, on the other hand, has been demonstrated in the ground and excited state by using steady-state as well as time-resolved spectroscopies, which were further complemented by microscopy.
[nsbp]
Carbon nanotubes and graphene are outstanding materials of the 21st century with a broad spectrum of applications. However, major challenges are faced such as the intrinsically low solubility of both sp2 carbon allotropes. To overcome this hurdle the potential of noncovalent functionalization is summarized with a special focus on the establishment of the perylene bisimide unit as aromatic anchor to the graphitic surface. Rational surfactant design is unmasked as the key to solubilization of the carbon allotropes, while at the same time tailoring their surface properties, or even electronic properties in a fully reversible fashion.
[nsbp]
 Mutual attraction: π–π bonding promotes electronic interactions between graphene and a π-conjugated perylene in a liquid dispersion.
Mutual attraction: π–π bonding promotes electronic interactions between graphene and a π-conjugated perylene in a liquid dispersion.
Herein, we report for the first time on the electronic communication between graphene with the perylene bisimide (PBI) when both are deposited on a surface or dispersed in homogeneous solution. This interaction is provided by the non-covalent binding of their conjugated π-systems.
[nsbp]
![]() The synthesis and characterization of three highly water-soluble perylenetetracarboxdiimide (“perylene bisimide”, PBI) dyes 1 and 2b is presented. The water solubility is provided by peripheral dendronization with 1G- and 2G-Newkome dendrons. The aggregation behaviour of these amphiphiles in water and that of their tert-butyl-protected precursor molecules 3 and 4 in organic solvents was investigated by UV/Vis- and fluorescence spectroscopy. The symmetric 1G-dendronized dye 1a forms aggregates more easily than its 2G-counterpart 1b. The asymmetric derivatives 2b reveals pronounced aggregation properties due to the presence of both a 2G-dendron and a flexible and non-bulky dodecyl substituent. The 1G-analogue 2a is insoluble in water as a result of insufficient overall hydrophilicity caused by only one 1G-Newkome dendron. Within the series of water-soluble perylenes 1 and 2b the asymmetric derivative 2b forms the most regularly shaped micelles in water (pH = 7.2) with a mean diameter of 16 nm as demonstrated by transition electron microscopy (TEM). The bola-amphiphiles 1a and 1b form a distribution of smaller aggregates on average with less defined shape. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2007)
The synthesis and characterization of three highly water-soluble perylenetetracarboxdiimide (“perylene bisimide”, PBI) dyes 1 and 2b is presented. The water solubility is provided by peripheral dendronization with 1G- and 2G-Newkome dendrons. The aggregation behaviour of these amphiphiles in water and that of their tert-butyl-protected precursor molecules 3 and 4 in organic solvents was investigated by UV/Vis- and fluorescence spectroscopy. The symmetric 1G-dendronized dye 1a forms aggregates more easily than its 2G-counterpart 1b. The asymmetric derivatives 2b reveals pronounced aggregation properties due to the presence of both a 2G-dendron and a flexible and non-bulky dodecyl substituent. The 1G-analogue 2a is insoluble in water as a result of insufficient overall hydrophilicity caused by only one 1G-Newkome dendron. Within the series of water-soluble perylenes 1 and 2b the asymmetric derivative 2b forms the most regularly shaped micelles in water (pH = 7.2) with a mean diameter of 16 nm as demonstrated by transition electron microscopy (TEM). The bola-amphiphiles 1a and 1b form a distribution of smaller aggregates on average with less defined shape. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2007)
[nsbp]
Supramolecular Chemistry
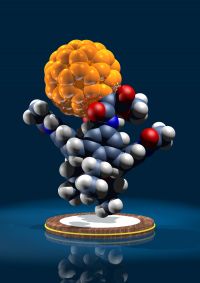
For the construction of molecular assemblies and materials of higher hierarchical order, we systematically employed non-covalent binding motifs such as hydrogen bonding, electrostatic bonding and hydrophobic interactions. For this purpose we design and synthesize new molecules, which carry the required recognition motifs in their periphery. Examples are precisely defined oligo-electrolytes with up 60 charges in their periphery or porpyrines, perylenes, fullerenes and carbon nanotubes that involve Hamilton receptor/cyanuric acid binding motifs. Of practical use are in particular layer-by-layer assembly architectures where positively and negatively charged chromophores are successively deposited on surfaces. In this way for example new photovoltaic devices can be designed.
Selected Publications
Publikationen
 A three-pronged approach has been used to design rational improvements in self-assembled monolayer field-effect transistors: classical molecular dynamics (MD) simulations to investigate atomistic structure, large-scale quantum mechanical (QM) calculations for electronic properties, and device fabrication and characterization as the ultimate goal. The MD simulations reveal the effect of using two-component monolayers to achieve intact dielectric insulating layers and a well-defined semiconductor channel. The QM calculations identify improved conduction paths in the monolayers that consist of an optimum mixing ratio of the components. These results have been used both to confirm the predictions of the calculations and to optimize real devices. Monolayers were characterized with X-ray reflectivity measurements and by electronic characterization of complete devices.
A three-pronged approach has been used to design rational improvements in self-assembled monolayer field-effect transistors: classical molecular dynamics (MD) simulations to investigate atomistic structure, large-scale quantum mechanical (QM) calculations for electronic properties, and device fabrication and characterization as the ultimate goal. The MD simulations reveal the effect of using two-component monolayers to achieve intact dielectric insulating layers and a well-defined semiconductor channel. The QM calculations identify improved conduction paths in the monolayers that consist of an optimum mixing ratio of the components. These results have been used both to confirm the predictions of the calculations and to optimize real devices. Monolayers were characterized with X-ray reflectivity measurements and by electronic characterization of complete devices.
[nsbp]
 A concept is elaborated of pairing electron donors and electron acceptors that share a common trait, wire-like features, as a powerful means to realize a new and versatile class of electron donor−acceptor nanohybrids. Important variables are fine-tuning (i) the complexation strength, (ii) the electron/energy transfer behavior, and (iii) the solubilities of the resulting architectures. In particular, a series of supramolecular porphyrin/fullerene hybrids assembled by the hydrogen bonding of Hamilton receptor/cyanuric acid motif has been realized. Putting the aforementioned variables into action, the association constants (Kass), as they were determined from 1H NMR and steady-state fluorescence assays, were successfully tweaked with values in the range of 104−105 M−1.
A concept is elaborated of pairing electron donors and electron acceptors that share a common trait, wire-like features, as a powerful means to realize a new and versatile class of electron donor−acceptor nanohybrids. Important variables are fine-tuning (i) the complexation strength, (ii) the electron/energy transfer behavior, and (iii) the solubilities of the resulting architectures. In particular, a series of supramolecular porphyrin/fullerene hybrids assembled by the hydrogen bonding of Hamilton receptor/cyanuric acid motif has been realized. Putting the aforementioned variables into action, the association constants (Kass), as they were determined from 1H NMR and steady-state fluorescence assays, were successfully tweaked with values in the range of 104−105 M−1.
[nsbp]
 A series of novel supramolecular architectures were built around a tin tetraphenyl porphyrin platform 6—functionalized by a 2-fold 1-ethyl-3-3-(3-dimethylaminopropyl)carbodiimide (EDC) promoted condensation reaction—and chiral depsipeptide dendrons of different generations 1—4. Here, implementation of a Hamilton receptor provided the necessary means to keep the constituents together via strong hydrogen bonding. Characterization of all architectures has been performed, including 4 which is the fourth generation, on the basis of NMR and photophysical methods. In particular, several titration experiments were conducted suggesting positive cooperativity, an assessment that is based on association constants that tend to be higher for the second binding step than for the first step. Importantly, molecular modeling calculations reveal a significant deaggregation of the intermolecular network of 6 during the course of the first binding step. As a consequence, an improved accessibility of the second Hamilton receptor unit in 6 emerges and, in turn, facilitates the higher association constants. The features of the equilibrium, that is, the dynamic exchange of depsipeptide dendrons 1−4 with fullerene 5, was tested in photophysical reference experiments. These steady-state and time-resolved measurements showed the tunable excited-state deactivations of these complexes upon photoexcitation.
A series of novel supramolecular architectures were built around a tin tetraphenyl porphyrin platform 6—functionalized by a 2-fold 1-ethyl-3-3-(3-dimethylaminopropyl)carbodiimide (EDC) promoted condensation reaction—and chiral depsipeptide dendrons of different generations 1—4. Here, implementation of a Hamilton receptor provided the necessary means to keep the constituents together via strong hydrogen bonding. Characterization of all architectures has been performed, including 4 which is the fourth generation, on the basis of NMR and photophysical methods. In particular, several titration experiments were conducted suggesting positive cooperativity, an assessment that is based on association constants that tend to be higher for the second binding step than for the first step. Importantly, molecular modeling calculations reveal a significant deaggregation of the intermolecular network of 6 during the course of the first binding step. As a consequence, an improved accessibility of the second Hamilton receptor unit in 6 emerges and, in turn, facilitates the higher association constants. The features of the equilibrium, that is, the dynamic exchange of depsipeptide dendrons 1−4 with fullerene 5, was tested in photophysical reference experiments. These steady-state and time-resolved measurements showed the tunable excited-state deactivations of these complexes upon photoexcitation.
[nsbp]
 A new modular concept for the self-assembly of electron donor−acceptor complexes is presented that ensures (i) fine-tuning the strength of the complexation, (ii) controlling the electronic coupling to impact electron and energy transfer processes, and (iii) high solubility of the corresponding hybrid architectures. This task has been realized through developing a series of porphyrin−fullerene donor−acceptor systems held together by a Hamilton-receptor-based hydrogen-bonding motif. In this context, novel libraries of C60 monoadducts (1) containing cyanuric acid side chains and of tetraphenylporphyrin derivatives (2) involving the complementary Hamilton-receptor unit were synthesized.
A new modular concept for the self-assembly of electron donor−acceptor complexes is presented that ensures (i) fine-tuning the strength of the complexation, (ii) controlling the electronic coupling to impact electron and energy transfer processes, and (iii) high solubility of the corresponding hybrid architectures. This task has been realized through developing a series of porphyrin−fullerene donor−acceptor systems held together by a Hamilton-receptor-based hydrogen-bonding motif. In this context, novel libraries of C60 monoadducts (1) containing cyanuric acid side chains and of tetraphenylporphyrin derivatives (2) involving the complementary Hamilton-receptor unit were synthesized.
[nsbp]
![Supramolecular Structure of 5-nm Spherical Micelles with D3 Symmetry Assembled from Amphiphilic [3:3]-Hexakis Adducts of C60](../files/_upload/Content/sonstiges/sphericalmielles.gif) Six molecules of an amphiphilic fullerene derivative make up the smallest persistent micelle detected so far (see picture, the six amphiphilic molecules are shown in different colors). These micelles can be used to systematically study the factors that determine the structural persistence of micelles and may lead to the design of tailor-made supramolecular containers.
Six molecules of an amphiphilic fullerene derivative make up the smallest persistent micelle detected so far (see picture, the six amphiphilic molecules are shown in different colors). These micelles can be used to systematically study the factors that determine the structural persistence of micelles and may lead to the design of tailor-made supramolecular containers.
[nsbp]
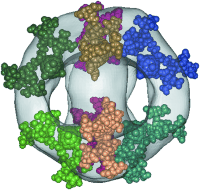 Exactly eight amphiphilic fullerene dendrimer molecules form a globular micelle spontaneously in aqueous solution. This supramolecular organization can be turned on and off by an external stimulus (pH). Owing to the remarkable structure persistence of the micelles, their three-dimensional structure could be determined from cryogenic transmission electron micrographic images and the molecular architecture was subsequently modeled (see picture).
Exactly eight amphiphilic fullerene dendrimer molecules form a globular micelle spontaneously in aqueous solution. This supramolecular organization can be turned on and off by an external stimulus (pH). Owing to the remarkable structure persistence of the micelles, their three-dimensional structure could be determined from cryogenic transmission electron micrographic images and the molecular architecture was subsequently modeled (see picture).
[nsbp]
Functionalization of Nanoparticles
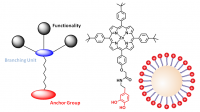
We are investigating the controlled chemical functionalization of inorganic nanoparticles (NP) such as ZnO-, TiO2-, Ag- and Au quantum dots and ZnO nanorods with organic building blocks. The latter include neutral and charged dendrimers, porphyrines and H-bonding architectures such as cyanuric acid derivatives and Hamiliton receptors. In this way we are able to improve the stability and dispersibility of the nanoparticles and control their supramolecular aggregation including layer-by-layer assembly on surfaces. The chemical derivatization of the nanoparticles with redox-active molecules and light-harvesting antennas is also important for their implemention into optoelectronic applications.
Selected Publications
Publikationen
 The current work addresses the understanding of the stabilization of nanoparticles in suspension. Specifically, we study ZnO in ethanol for which the influence of particle size and reactant ratio as well as surface coverage on colloidal stability in dependence of the purification progress was investigated. The results revealed that the well-known ζ-potential determines not only the colloidal stability but also the surface coverage of acetate groups bound to the particle surface. The acetate groups act as molecular spacers between the nanoparticles and prevent agglomeration. Next to DLVO calculations based on the theory of Derjaguin, Landau, Verwey and Overbeek using a core–shell model we find that the stability is better understood in terms of dimensionless numbers which represent attractive forces as well as electrostatic repulsion, steric effects, transport properties, and particle concentration.
The current work addresses the understanding of the stabilization of nanoparticles in suspension. Specifically, we study ZnO in ethanol for which the influence of particle size and reactant ratio as well as surface coverage on colloidal stability in dependence of the purification progress was investigated. The results revealed that the well-known ζ-potential determines not only the colloidal stability but also the surface coverage of acetate groups bound to the particle surface. The acetate groups act as molecular spacers between the nanoparticles and prevent agglomeration. Next to DLVO calculations based on the theory of Derjaguin, Landau, Verwey and Overbeek using a core–shell model we find that the stability is better understood in terms of dimensionless numbers which represent attractive forces as well as electrostatic repulsion, steric effects, transport properties, and particle concentration.
[nsbp]
 A new concept for the efficient synthesis of cationic dendrons, 4-tert-butyl-1-(3-(3,4-dihydroxybenzamido)benzyl)pyridinium bromide (17), 1,1′-(5-(3,4-dihydroxybenzamido)-1,3-phenylene)bis(methylene)bis(4-tert-butylpyridinium) bromide (18), N1,N7-bis(3-(4-tert-butyl-pyridium-methyl)phenyl)-4-(3-(3-(4-tert-butyl-pyridinium-methyl)phenyl-amino)-3-oxopropyl)-4-(3,4-dihydroxybenzamido)heptanediamide tribromide (19), and N1,N7-bis(3,5-bis(4-tert-butyl-pyridium-methyl)phenyl)-4-(3-(3,5-bis(4-tert-butyl-pyridinium-methyl)phenylamino)-3-oxopropyl)-4-(3,4-dihydroxybenzamido)heptanediamide hexabromide (20), and their facile binding to zinc oxide (ZnO) nanostructures is introduced.
A new concept for the efficient synthesis of cationic dendrons, 4-tert-butyl-1-(3-(3,4-dihydroxybenzamido)benzyl)pyridinium bromide (17), 1,1′-(5-(3,4-dihydroxybenzamido)-1,3-phenylene)bis(methylene)bis(4-tert-butylpyridinium) bromide (18), N1,N7-bis(3-(4-tert-butyl-pyridium-methyl)phenyl)-4-(3-(3-(4-tert-butyl-pyridinium-methyl)phenyl-amino)-3-oxopropyl)-4-(3,4-dihydroxybenzamido)heptanediamide tribromide (19), and N1,N7-bis(3,5-bis(4-tert-butyl-pyridium-methyl)phenyl)-4-(3-(3,5-bis(4-tert-butyl-pyridinium-methyl)phenylamino)-3-oxopropyl)-4-(3,4-dihydroxybenzamido)heptanediamide hexabromide (20), and their facile binding to zinc oxide (ZnO) nanostructures is introduced.
[nsbp]
 Novel types of binding motives have been investigated within the context of sensitizing ZnO-based dye-sensitized solar cells with metalloporphyrins. In particular, a complementary class of metalloporphyrin has been synthesized to probe the impact of a face-to-edge/orthogonal versus face-to-face/parallel orientation of the metalloporphyrin with respect to ZnO and has been compared to TiO2-based dye-sensitized solar cells. Our studies provide a deep and detailed understanding of the individual electron-transfer processes at the ZnO/metalloporphyrin interface, that is, electron injection, recombination, and dye regeneration, by means of steady-state and time-resolved techniques. Interestingly, we found that for our novel ZnO/metalloporphyrin systems, the injection efficiencies are close to unity, despite their long, nonconjugated anchoring group length.
Novel types of binding motives have been investigated within the context of sensitizing ZnO-based dye-sensitized solar cells with metalloporphyrins. In particular, a complementary class of metalloporphyrin has been synthesized to probe the impact of a face-to-edge/orthogonal versus face-to-face/parallel orientation of the metalloporphyrin with respect to ZnO and has been compared to TiO2-based dye-sensitized solar cells. Our studies provide a deep and detailed understanding of the individual electron-transfer processes at the ZnO/metalloporphyrin interface, that is, electron injection, recombination, and dye regeneration, by means of steady-state and time-resolved techniques. Interestingly, we found that for our novel ZnO/metalloporphyrin systems, the injection efficiencies are close to unity, despite their long, nonconjugated anchoring group length.
[nsbp]
 Time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron spectroscopy (XPS) were used to study monolayers (ML) and thick films of porphyrin Zn-TESP (C67H75N5O5SiZn) attached to titanium dioxide (TiO2) substrates via silanization. Films on ideal hydroxyl-terminated silicon (SiO2) surfaces were used for comparison. ToF-SIMS and XPS spectra show that the type of adsorption varies depending on the thickness of the organic film, the preparation temperature, and the adsorption time. We show that the intensity of a molecular peak at mass 1121.5 u in ToF-SIMS can be used as a direct measure of the ratio of chemisorption/physisorption of Zn-TESP. On TiO2, the amount of chemisorbed porphyrin can be increased by increasing the reaction temperature and time during the silanization process.
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron spectroscopy (XPS) were used to study monolayers (ML) and thick films of porphyrin Zn-TESP (C67H75N5O5SiZn) attached to titanium dioxide (TiO2) substrates via silanization. Films on ideal hydroxyl-terminated silicon (SiO2) surfaces were used for comparison. ToF-SIMS and XPS spectra show that the type of adsorption varies depending on the thickness of the organic film, the preparation temperature, and the adsorption time. We show that the intensity of a molecular peak at mass 1121.5 u in ToF-SIMS can be used as a direct measure of the ratio of chemisorption/physisorption of Zn-TESP. On TiO2, the amount of chemisorbed porphyrin can be increased by increasing the reaction temperature and time during the silanization process.
[nsbp]
 Stable ZnO nanoparticles suitable for further surface functionalization were synthesized in the liquid phase from homogeneous ethanolic solutions of the precursors lithium hydroxide and zinc acetate. It was found that the growth of the particles was governed by temperature as well as the presence of the reaction byproduct lithium acetate during the aging process. In particular, the reaction could be almost completely arrested by removal of this byproduct. The “washing” consisted of repeated precipitation of the ZnO particles by addition of alkanes such as heptane, removal of the supernatant, and redispersion in ethanol. Furthermore, the surface of the colloidal ZnO nanoparticles was successfully modified by catechol-anchoring group containing dye molecules for the study of photochemical properties.
Stable ZnO nanoparticles suitable for further surface functionalization were synthesized in the liquid phase from homogeneous ethanolic solutions of the precursors lithium hydroxide and zinc acetate. It was found that the growth of the particles was governed by temperature as well as the presence of the reaction byproduct lithium acetate during the aging process. In particular, the reaction could be almost completely arrested by removal of this byproduct. The “washing” consisted of repeated precipitation of the ZnO particles by addition of alkanes such as heptane, removal of the supernatant, and redispersion in ethanol. Furthermore, the surface of the colloidal ZnO nanoparticles was successfully modified by catechol-anchoring group containing dye molecules for the study of photochemical properties.
[nsbp]
