PD Dr. Konstantin Amsharov

Nachwuchsgruppenleiter
| Raum: | 02.137 |
| Tel: | +49 (0) 9131 85 65507 |
| E-Mail: | konstantin.amsharov@fau.de |
Kurzbiographie
Dr. Konstantin Yu. Amsharov studied chemistry at Sankt-Petersburg State University in Sankt-Petersburg. In 2002 he received his Ph.D from the Institute of Macromolecular Compounds, Russian Academy of Science. From 2002 to 2004 he was a postdoctoral fellow in the group of Prof. Shamanin (Institute of Macromolecular Compounds, Russian Academy of Science). In 2005, he moved to Max-Planck Institute for Solid State Research, Stuttgart where he worked with Prof. Martin Jansen on several projects in the area of fullerene chemistry and synthesis of higher and non-IPR fullerenes. From 2009 to 2012 he received his habilitation in Organic chemistry in the subject “Direct synthesis of carbon based nanostructures: buckybowls, higher fullerenes and nanotubes” at the Institute of Organic Chemistry, University of Stuttgart, Germany. From 2005 to 2013 he was a Group Leader of the “Fullerene group” in the department of Prof Martin Jansen (Max-Planck Institute for Solid State Research, Stuttgart). Since 2014 he is leading a junior research group (Heisenberg program) at FAU Erlangen-Nürnberg.
Mitarbeiter
| Dr. Ekaterina Roshchina | kateryna.roshchyna@fau.de |
| Olena Papaianina, MSc | olena.papaianina@fau.de |
| Jorg Tomada, Msc | joerg.tomada@fau.de |
| Arber Uka, Msc | arber.uka@fau.de |
| Stanislav Shkolnikov, BSc | stanislav.shkolnikov@fau.de |
| Ann-Kristin Steiner, BSc |
Motivated undergraduate students with an interest in our research topics are encouraged to join our group.
Forschungsbereich
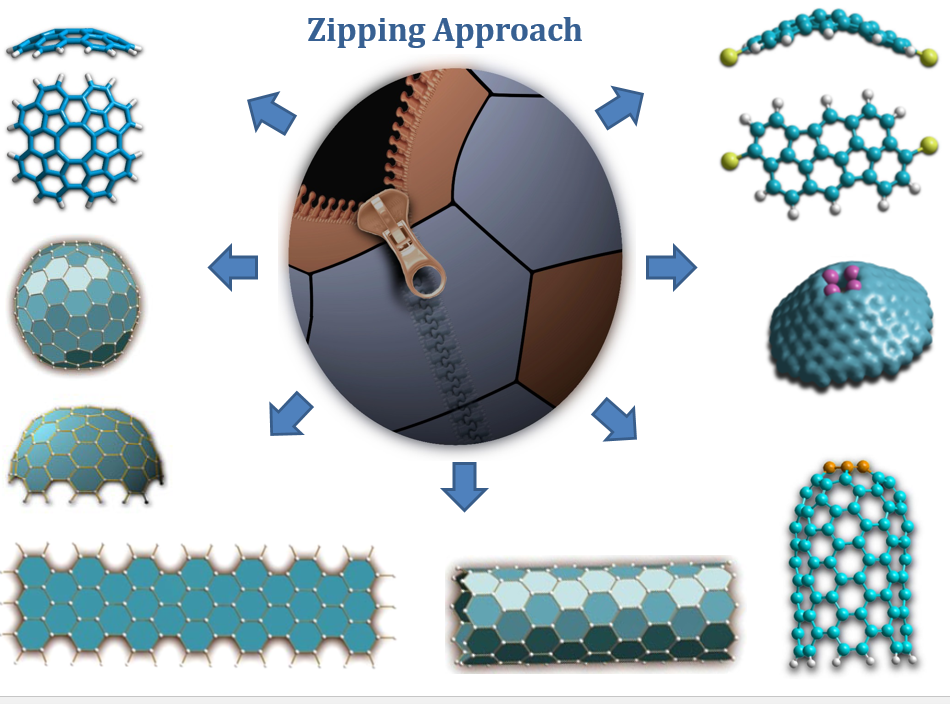
Our research group has its main focus on the rational synthesis of sp2-carbon based nanostructures which includes the rational synthesis of higher and non-classical fullerenes, geodesic polycyclic hydrocarbons, buckybowls, graphene nanoribbons, nanographenes carbon nanotubes and other related system. Our synthetic strategy is based on the synthesis of the precursor molecules - polycyclic aromatic hydrocarbons, containing the carbon framework required for the formation of the target nanostructure. The respective precursor molecule can be “rolled up” to the desired carbon nanostructure by intramolecular Aryl-Aryl domino-coupling. For this purpose specially designed precursor molecules are synthesized and investigated for controlled synthesis of the respective carbon nanostructures. The characteristic feature of the approach is a zipper mechanism of cyclization (regiospecific condensation in a domino fashion) by which the regiospecifity of each condensation step is unambiguously predefined by the specially “designed” precursor structure. Our main goal is to find and to develop alternative synthetic approaches suitable for preparative production of various carbon allotrope in isomerically pure form.
ausgewählte Publikationen
Publikationen
Thermally activated γ-aluminium oxide was found to be very effective for C–F bond activation in trifluoromethylated arenes. Depending on the activation degree the respective arenes can be converted either to cyclic ketones or to the respective carboxylic acids with good to excellent yields.The high selectivity of the reaction is a consequence of an effective encapsulation of the reagent in to the alumina nanopore environment. In general our finding demonstrates that the CF3 functionality can be reconsidered as synthetically useful functionality and an effective synthon for carboxyl groups.
[nsbp]

We have shown that specially designed polycyclic aromatic hydrocarbons (PAHs) can be quantitatively converted to the “preprogrammed” ultra-short singly-capped nanotubes bearing several CNT segments. The tubes obtained by this route are already bonded to the metal and can be used directly for the SWCNT fabrication on the same metal surface. We have demonstrated that CNT seeds are active in tube growth initiation and can be grown to isomerically pure SWCNTs by epitaxial elongation under Chemical Vapor Deposition (CVD) condition. For the first time the uncontrolled CNT nucleation has been avoided and predefined isomerically pure and defect free SWCNTs were effectively fabricated.
[nsbp]

A specially fluorinated C60 fullerene precursor was converted to the target C60 cage by laser ionisation via highly selective HF elimination without any detectable side reactions and undesired fragmentations. The fully selective transformation to the target fullerene has been unambiguously demonstrated using a 13C-labeled precursor. In general the findings open new horizons for the synthesis of new carbon-based nanomaterials, which cannot be obtained by any conventional alternative method.
[nsbp]
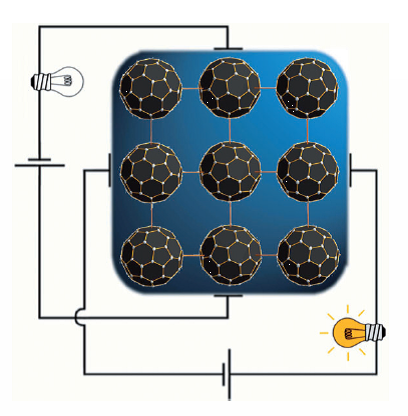
We have synthesized and characterized single crystals of the fulleride based 1D electric conductor (KC60(THF)5·2THF) in which C60 anions are highly ordered in 2D corrugated layers. The combination of the counter alkali metal cation coordinated with solvent ligands prevents the dimerization of C60·- and the conductivity loss over a large temperature range. The spatial separation of particular fullerene anions kept their orbital correlation resulting in the 1D conductivity in the 2D layer with counterintuitive anisotropy. On the base of experimental ESR and SQUID data and X-ray analysis we have demonstrated that conduction electrons move freely only along the C60-channels with larger intercentroid separation whereas the channels with shorter C60-C60 contacts in the same layer are nonconductive.
[nsbp]
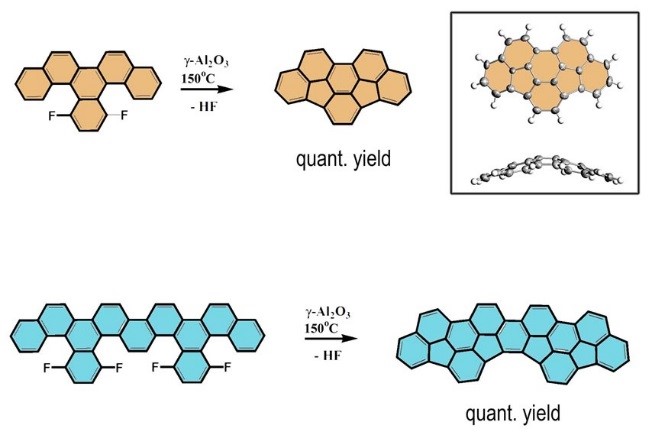
Building bowls: An effective intramolecular aryl–aryl coupling is the key step in the rational synthesis of carbon based nanostructures. We have found that such a process can be embodied very efficiently through quantitative HF elimination on active Al2O3. The process is characterized by an unprecedentedly high chemoselectivity and regiospecificity allowing the facile synthesis of extended bowl shaped hydrocarbons in multi-gram scale.
[nsbp]
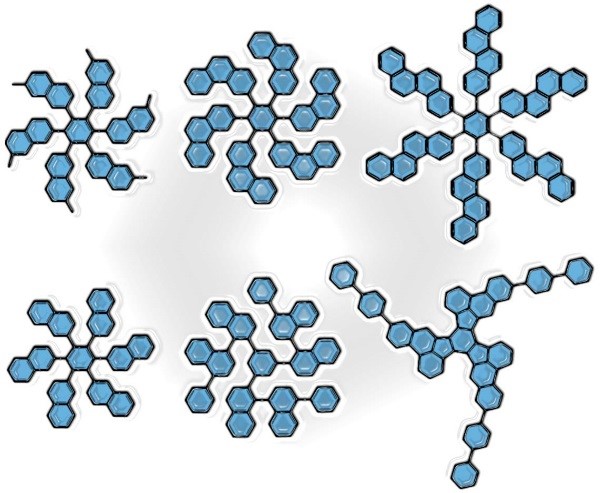
Synthesis of polycyclic aromatic hydrocarbons possessing a carbon connectivity required for the generation of extended hemispherical buckybowls via intramolecular cyclodehydrogenation is a key prerequisity for the rational synthesis of isomerically pure single-walled carbon nanotubes (SWCNTs). We have developed several approaches to the precursors for the rational synthesis of isomerically pure armchair, zigzag and chiral single-walled carbon nanotubes (SWCNTs). The suggested synthesis strategy provides further options for the construction of precursors for virtually any possible SWCNT chiralities.
[nsbp]
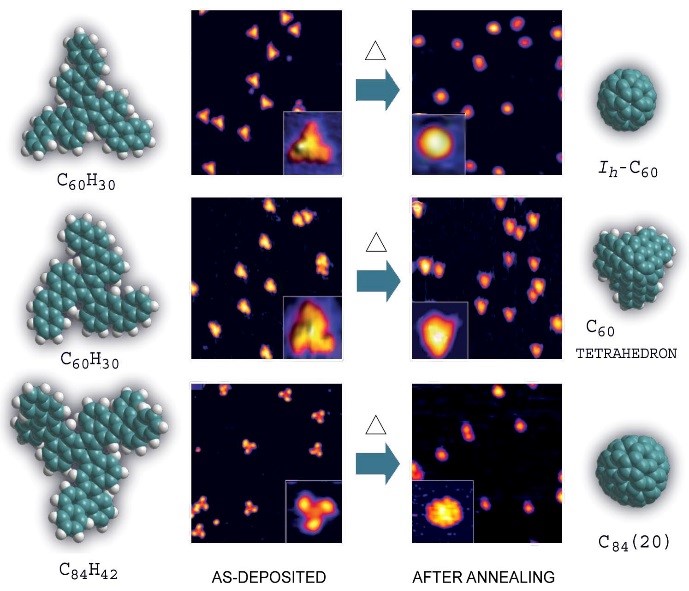
A Pt surface-catalyzed cyclodehydrogenation reaction enabled selective formation of various carbon based nanostructures from specially “preprogrammed” polycyclic organic precursors. As no C-C bond rearrangement occurred during the reaction, only specifically designed precursors gave the desired carbon nanostructure (see picture).This efficient and selective condensation process opens new horizons in the directed synthesis of fullerenes and related structures. On the basis of these results, for the first time we successfully proceeded the rational synthesis of a higher fullerene C84(20) by folding of the respective C84H42 precursor.
[nsbp]